Catalizadores a base de platino en varios soportes de carbono y polímeros conductores para aplicaciones de celdas de combustible de metanol directo:una revisión
Resumen
Los metales de nanopartículas a base de platino (Pt) han recibido una atención considerable y son los catalizadores más populares para la pila de combustible de metanol directo (DMFC). Sin embargo, el alto costo de los catalizadores de Pt, la oxidación cinética lenta y la formación de moléculas intermedias de CO durante la reacción de oxidación del metanol (MOR) son desafíos importantes asociados con los catalizadores de Pt de un solo metal. Estudios recientes se centran en el uso de aleaciones de Pt, como metales Fe, Ni, Co, Rh, Ru, Co y Sn, o materiales de soporte de carbono para mejorar el rendimiento catalítico de Pt. En los últimos años, los catalizadores de aleación de Pt y Pt apoyados en un gran potencial de materiales de carbono como MWCNT, CNF, CNT, CNC, CMS, CNT, CB y grafeno han recibido un interés notable debido a sus importantes propiedades que pueden contribuir al excelente MOR. y rendimiento DMFC. Este artículo de revisión resume el desarrollo de las aleaciones anteriores y los materiales de soporte relacionados para reducir el uso de Pt, mejorar la estabilidad y un mejor rendimiento electrocatalítico de Pt en DMFC. Finalmente, se presenta una discusión de cada catalizador y soporte en términos de morfología, actividad electrocatalítica, características estructurales y el desempeño de su pila de combustible.
Introducción
La tecnología de pilas de combustible ha ganado una atención generalizada en todo el mundo. Las pilas de combustible (FC) son una tecnología alternativa prometedora de generación de energía que convierte la energía química en energía eléctrica a través de una reacción electroquímica [1, 2]. Además, para la tecnología de pilas de combustible, el enfoque principal en la tecnología de pilas de combustible es generar una producción de bajo costo, logrando así un rendimiento potente del sistema de pilas de combustible y descubriendo materiales duraderos. No obstante, los problemas comunes que surgen en la tecnología actual de pilas de combustible son que los sistemas implican altos costes intrínsecos y poca durabilidad [1]. A pesar de su promesa como celda de combustible, las celdas de combustible de metanol directo (DMFC) tienen desafíos y limitaciones, lo que lleva a los investigadores a estudiar métodos para mejorar la eficiencia y el rendimiento de DMFC. Se han identificado muchos problemas con DMFC y siguen sin resolverse, incluido el cruce de combustible de metanol del electrodo del ánodo al electrodo del cátodo [3,4,5] rendimiento deficiente causado por la velocidad cinética lenta, la inestabilidad del catalizador y la gestión térmica y del agua. [6, 7, 8].
Recientemente, se han realizado numerosas investigaciones sobre pilas de combustible, que incluyen DMFC, pila de combustible de membrana de intercambio de protones (PEMFC), pila de combustible de óxido sólido (SOFC), etc., que son tecnologías populares de pila de combustible. Como nueva fuente de energía, los DMFC se pueden utilizar para aplicaciones móviles y estacionarias [9, 10]. Se han logrado muchos avances en la investigación en el campo de las pilas de combustible. Entre las pilas de combustible, los DMFC se han estudiado ampliamente en los últimos años [11,12,13,14,15,16] debido a sus muchas ventajas, como alta densidad de potencia, facilidad de manejo del combustible, facilidad de carga y bajo impacto ambiental. impacto [17, 18]. Sin embargo, varios desafíos técnicos para la comercialización de DMFC siguen sin resolverse, incluido el cruce de metanol, las bajas velocidades de reacción química y el envenenamiento del catalizador. Sin embargo, los DMFC todavía han recibido la atención de muchos investigadores y se han convertido en las pilas de combustible más populares debido a su funcionamiento a baja temperatura (los sistemas DMFC funcionan a 373 K). Debido a las ventajas de DMFC de alta eficiencia energética y sistema de arranque rápido, la tecnología DMFC es muy adecuada para ser aplicada como fuentes de energía residenciales, baterías en dispositivos móviles y como combustible para vehículos [19,20,21,22]. Además, el concepto de DMFC podría estudiarse más a fondo para encontrar fuentes de combustible alternativas, como el gas natural y la biomasa, así como la fermentación de productos agrícolas para producir etanol, con el fin de minimizar la dependencia de fuentes de energía inseguras [14].
En DMFC, el lado del ánodo se suministra con una solución de metanol que se someterá a electrooxidación a dióxido de carbono (CO 2 ) a través de la reacción a continuación:
$$ {\ mathrm {CH}} _ 3 \ mathrm {OH} + {\ mathrm {H}} _ 2 \ to {\ mathrm {CO}} _ 2 + 6 {\ mathrm {H}} ^ {+} + 6 { \ mathrm {e}} ^ {\ hbox {-}} $$ (1)Mientras que en el lado del cátodo el protón, el oxígeno (del aire) se reduce a agua:
$$ 3/2 \ {\ mathrm {O}} _ 2 + 6 {\ mathrm {H}} ^ {+} + 6 \ {\ mathrm {e}} ^ {\ hbox {-}} \ a 3 {\ mathrm {H}} _ 2 \ mathrm {O} $$ (2)La reacción de DMFC de la ecuación neta se puede resumir de la siguiente manera:
$$ {\ mathrm {CH}} _ 3 \ mathrm {OH} +3/2 {\ mathrm {O}} _ 2 \ to {\ mathrm {CO}} _ 2 + 2 {\ mathrm {H}} _ 2 \ mathrm { O} $$ (3)En los sistemas DMFC, hay dos tipos de modos DMFC:modos activo y pasivo [23, 24, 25]. En un sistema DMFC activo, la corriente de salida de la pila de DMFC se recircula a través del control de circuito cerrado de la alimentación de metanol líquido. Mientras tanto, el metanol líquido en la corriente del ánodo es controlado por un sensor de concentración de metanol que juega un papel importante al proporcionar suficiente inyección de metanol y agua adicionales para restaurar este combustible en función de la concentración objetivo. Hay varios tipos de sensores de concentración de metanol que se utilizan en el sistema DMFC para controlar y mantener la concentración de la alimentación de metanol [17]. Normalmente, el metanol líquido se suministra al lado del ánodo mediante una bomba peristáltica, mientras que el aire circundante que contiene oxígeno se suministra al lado del cátodo mediante un soplador o ventilador [16]. En un modo pasivo del sistema DMFC, el metanol líquido se alimenta continuamente al sistema. Este concepto pasivo es muy atractivo para el sistema DMFC [26,27,28]. El concepto de pasivo significa que el sistema funciona de forma totalmente autónoma sin ningún dispositivo de soporte. El concepto de DMFC pasivo significa que el sistema funciona de forma totalmente autónoma sin la ayuda de un dispositivo externo para bombear metanol y soplar aire en la chimenea. En el modo pasivo del sistema DMFC, la capa de catalizador será suministrada por metanol y oxígeno a medida que reacciona. Durante la reacción de oxidación del metanol (MOR), CO 2 y el agua se eliminará de la celda por medios pasivos, es decir, difusión, convección natural, acción capilar, etc. [20]. El DMFC en modo pasivo parece más ventajoso en comparación con el DMFC en modo activo en términos de diseño más simple, compacto y de bajo costo. El diseño y los controles complejos del sistema pueden ser una desventaja del DMFC en modo activo [21]. Desde el aspecto de usos prácticos, el modo activo DMFC parece ser más apropiado en sistemas de alta potencia, mientras que el modo pasivo DMFC es más adecuado para ser utilizado en requisitos de baja potencia [22].
La Figura 1 muestra la configuración y el diseño de un DMFC de celda única. Una pila de DMFC de celda única consta de un conjunto de electrodo de membrana (MEA) de cinco capas intercalado por dos placas que son ánodo y cátodo. En el lado del ánodo, el metanol líquido (que contiene metanol y agua desionizada) y el metanol absoluto fluyen al canal mediante una bomba peristáltica. En el lado del cátodo, un rotámetro bombea aire a la celda de combustible. El controlador de temperatura en la pila DMFC se utiliza para mantener la temperatura de trabajo en la celda mediante un dispositivo de calentamiento suplementario. El dispositivo de carga electrónico se utiliza para cambiar la densidad de corriente a diferentes niveles y medir los valores correspondientes del voltaje. El rendimiento de la celda es monitoreado por una estación de trabajo electroquímica, mientras que la producción de CO 2 ya que el producto final de la reacción general se mide mediante un CO 2 detector de concentración [23]. En la celda de combustible de metanol directo, hay varios parámetros operativos importantes que deben considerarse durante el estudio experimental, que son (i) temperatura de trabajo, (ii) concentración de metanol y (iii) tasas de flujo de entrada de la solución de metanol de alimentación y aire [23] . La Figura 2a, b muestra el modo activo y pasivo de DMFC, respectivamente.
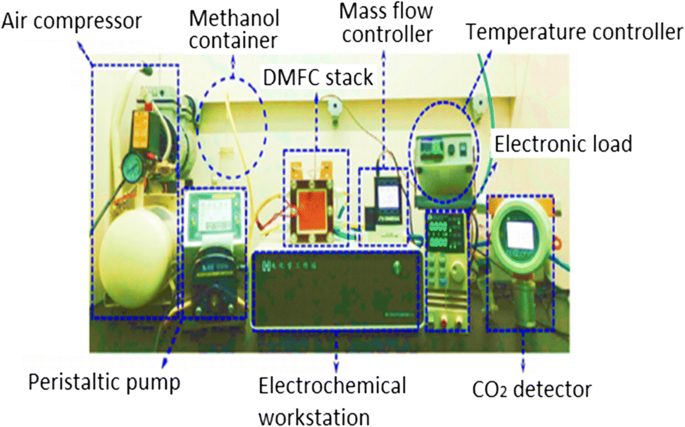
Una configuración experimental general para DMFC de celda única [23]
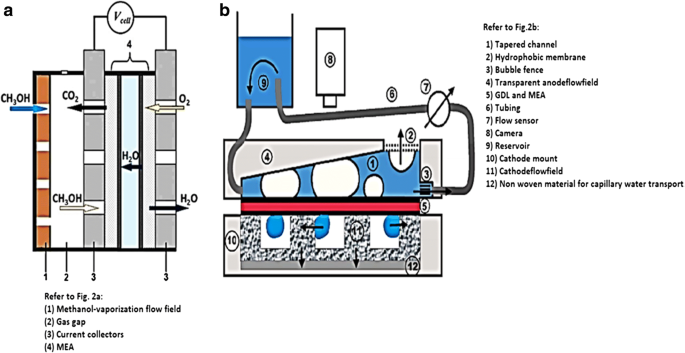
Diagrama esquemático de a modo activo [24] y b modo pasivo [25] de DMFC
Esta revisión se centrará en los avances recientes en las investigaciones y desarrollos del soporte de catalizador basado en el catalizador de Pt como catalizador noble en DMFC. Incluimos la actividad de catalizadores basados en Pt combinados con aleaciones, metales, metales de transición, carburos metálicos, nitruros metálicos y diversas especies carbonáceas, como grafeno / óxido de grafeno (G / GO), nanotubos de carbono (CNT), nanofibras de carbono ( CNF), nanobobina de carbono (CNC), negro de carbono (CB), nanotubos de carbono de paredes múltiples (MWCNT) y soportes mesoporosos de carbono (CMS), así como polímeros conductores, como polianilina (PANi) y polipirrol (Ppy) como soporte. material. Se pueden aplicar muchos métodos de síntesis para preparar catalizadores basados en Pt. Los métodos más comunes aplicados para obtener partículas de Pt a nanoescala son la impregnación [29,30,31,32,33,34], técnicas hidrotermales [35,36,37,38,39,40,41], microemulsión [42,43, 44,45] y reducción [46, 47]. Generalmente, el método de preparación podría afectar la morfología y el tamaño de las partículas de catalizador; por lo tanto, la selección del método para la síntesis del catalizador es muy importante.
Rendimiento de varios tipos de catalizadores basados en Pt
Durante la última década, muchos investigadores han centrado su investigación en el desarrollo de electrocatalizadores con el fin de mejorar su actividad electrocatalítica en metanol MOR para el sistema DMFC [37, 38]. El platino (Pt) es un catalizador de un solo metal que muestra una actividad catalítica significativamente alta para el MOR. Sin embargo, el Pt puro solo en un sistema DMFC puede ser fácilmente envenenado por las especies intermedias, es decir, el monóxido de carbono (CO), y el alto costo del catalizador de Pt limita su aplicación comercial como electrocatalizador, lo que reduce la velocidad cinética de oxidación del metanol. en el sistema DMFC [48,49,50]. Estos tres puntos son los principales obstáculos y limitaciones de usar Pt solo como electrocatalizador para DMFC. Sin embargo, para superar estos obstáculos, se han realizado varios estudios para sintetizar electrocatalizadores de aleación basados en Pt para lograr un mejor rendimiento electrocatalítico con menos uso de Pt [11, 47, 51, 52]. Normalmente, el tamaño medio de las partículas de Pt y su morfología se pueden determinar mediante análisis de micrografía de emisión de barrido (SEM) o micrografía electrónica de transmisión (TEM), que son los métodos más comunes en el campo de la catálisis que se pueden utilizar para caracterizar las propiedades físicas de los electrocatalizadores. La Tabla 1 muestra los tamaños de partícula promedio de las partículas de Pt con diferentes métodos de síntesis, propiedades y sus rendimientos.
El PtRu bimetálico se considera el catalizador más activo debido a su mecanismo bifuncional y los efectos del ligando [48, 53]. El PtRu se convierte en una interesante aleación de catalizador, y se ha utilizado hasta el día de hoy con muchos soportes de carbono. Sin embargo, el efecto toxicológico de la adición de rutenio (Ru) es incierto [49]. Por lo tanto, se ha realizado una investigación sobre aleaciones menos costosas que mezclan Pt con otros metales no preciosos [49,50,51,52, 54,55,56,57], como se explica en la sección "Rendimiento de las aleaciones a base de Pt".
Rendimiento de las aleaciones basadas en Pt
Arico y col. [35] encontró que se han realizado numerosos estudios para aumentar la actividad catalítica de los catalizadores de Pt en el MOR. En muchos estudios, la relación Pt-Ru óptima se ha identificado como 1:1, y los tamaños de partículas en la escala nanométrica son el tamaño ideal para mejorar la utilización del catalizador. Sin embargo, Shi et al. [38] identificaron que 3:2 era la proporción óptima de Pt-Ru en sus experimentos para mejorar la actividad catalítica de MOR. Aparte de eso, la actividad electrocatalítica para la actividad de electrooxidación del metanol también puede incrementarse si las partículas del electrocatalizador de PtRu son partículas de tamaño nanométrico en el rango de 2 a 4 nm. Paulas y col. [39] estuvo de acuerdo con esta declaración. Como sabemos, el Pt muestra una alta reactividad hacia el combustible de metanol, lo que hace que el metal Pt sea un electrocatalizador ideal para el electrodo de ánodo en el sistema DMFC. Sin embargo, durante el MOR del catalizador de Pt, se formará monóxido de carbono (CO), es decir, la especie intermedia, en la superficie de las partículas de Pt, que envenena así la superficie del catalizador [58, 59, 60, 61]. Por tanto, se necesitan algunos esfuerzos para superar el problema relacionado con la formación de especies venenosas en la superficie de las partículas de Pt, de modo que no cubran las áreas del sitio activo de Pt. Generalmente, las aleaciones binarias, como PtRu [62,63,64,65,66], PtRh [67,68,69,70,71], PtAu [72,73,74], PtSn [62, 63, 75, 76,77], PtNi [64,65,66,67,78,69], PtCo [70, 71, 78,79,80] y PtFe [81,82,83,84,85], se emplean con frecuencia. como combinaciones de electrocatalizador para el electrodo de ánodo en el sistema DMFC. Se cree que las adiciones de estos metales, como rutenio (Ru), estaño (Sn) y rodio (Rh), producen una mayor actividad catalítica.
La incorporación de níquel (Ni) en un catalizador a base de platino proporciona un rendimiento superior para MOR y DMFC. En una investigación más reciente, Guerrero-Ortega y colaboradores explican que la adición de Ni en el soporte de Pt-Vulcan promueve un incremento importante en la corriente farádica durante MOR de un orden de magnitud, aunque el uso de Pt es menor en el catalizador bimetálico [ 55]. Sus resultados experimentales también sugirieron que la adición de Ni promueve algunas modificaciones estructurales y electrónicas que mejoran un mejor desempeño de reacción en la interfaz del electrodo. En otro trabajo, la incorporación de Au en la aleación de Pt mejoró las actividades electrocatalíticas debido a la estructura electrónica cambiante y la mejora del área electroquímicamente activa (ECSA) [47]. Mientras que, la adición de estaño (Sn) a la aleación a base de Pt mostró un incremento en la actividad electrocatalítica, que está fuertemente influenciada por la incorporación de Sn en su sistema de aleación y formas oxidadas, impulsando la reacción más fácilmente debido al menor potencial de oxidación [ 56]. Además, la adición de cobalto (Co) a la aleación a base de Pt mejoró en gran medida las propiedades catalíticas del catalizador de PtCo (1:9) / rGO, que resultó ser diez veces mayor que el de Pt / rGO [51]. El aumento en la densidad de corriente se atribuye a una mayor dispersión de nanopartículas de PtCo en la naturaleza hidrófila del soporte rGO que promueve la activación del agua y conduce a oxidar los anuncios de CO en los sitios de Pt. Además, de acuerdo con el mecanismo bifuncional del Co, promueve el H 2 Activación de O creando más iones -OH y otros O 2 -especies que contienen para oxidar CO- especies intermedias en el sitio de Pt [57]. Este mecanismo bifuncional de Co también podría usarse para otros metales de transición catalíticos hacia MOR. El mecanismo de oxidación catalítica de las especies de CO a CO 2 en presencia de catalizadores de PtCo se puede resumir de la siguiente manera:
$$ \ mathrm {Pt} + {\ mathrm {CH}} _ 3 \ mathrm {OH} \ to \ mathrm {Pt} \ hbox {-} {\ mathrm {CO}} _ {\ mathrm {ads}} + 4 {\ mathrm {H}} ^ {+} + 4 {\ mathrm {e}} ^ {\ hbox {-}} $$ (4) $$ \ mathrm {Co} + {\ mathrm {H}} _ 2 \ mathrm {O} \ to \ mathrm {Co} {\ left (\ mathrm {OH} \ right)} _ {\ mathrm {ads}} + {\ mathrm {H}} ^ {+} + {\ mathrm {e }} ^ {\ hbox {-}} $$ (5) $$ {\ mathrm {PtCO}} _ {\ mathrm {ads}} + \ mathrm {Co} {\ left (\ mathrm {OH} \ right) } _ {\ mathrm {ads}} / {\ mathrm {CO}} _ 2+ \ mathrm {Pt} + \ mathrm {Co} + {\ mathrm {H}} ^ {+} + {\ mathrm {e}} ^ {\ hbox {-}} $$ (6)Además, Löffler et al. [86] sintetizó con éxito PtRu como catalizador de ánodo para DMFC mediante el cual se produjo el electrocatalizador más activo para la electrooxidación de metanol a aproximadamente 50 at.% De Ru. Mientras tanto, Dinh et al. informó [87] que PtRu con una proporción de PtRu 1:1 tiene un comportamiento metálico más fuerte y una mayor actividad electrocatalítica para la oxidación del metanol (MOR). El rendimiento está relacionado con estos dos factores principales:(i) área de superficie del catalizador maximizada y (ii) superficie del catalizador con un número máximo de sitios de aleación de metal de relación atómica cercana a 1:1. Ambos grupos también mostraron un alto nivel. Basado en el mecanismo bifuncional, Aricò et al. [58] y Goodenough et al. [62] sugirió que las especies intermedias de CO que se formaron en los sitios de superficie activa de Pt se pueden oxidar a dióxido de carbono (CO 2 ) por átomos de oxígeno activo formados en los elementos secundarios, por ejemplo, Ru, Sn y Mo, en la región de potencial inferior. La Tabla 1 resume el desempeño de varios tipos de catalizadores de aleación de Pt llevados a cabo por investigadores para MOR. De acuerdo con el mecanismo bifuncional [88,89,90], el MOR en catalizadores de aleación de PtRu soportados se puede resumir en la siguiente ecuación. Pt es un catalizador más activo para la adsorción de metanol que Ru. Por lo tanto, la reacción general en los electrocatalizadores PtRu para la reacción de oxidación del metanol obedece al mecanismo bifuncional.
$$ \ mathrm {Pt} + {\ mathrm {CH}} _ 3 \ mathrm {OH} \ to \ mathrm {Pt} \ hbox {-} {\ mathrm {CH}} _ 3 \ mathrm {OH} \ mathrm {anuncios } \ to \ mathrm {Pt} \ hbox {-} {\ mathrm {CO} \ mathrm {H}} _ {\ mathrm {ads}} \ to 3 \ mathrm {H} +3 \ mathrm {e} \ hbox {-} \ to \ mathrm {Pt} \ hbox {-} {\ mathrm {CO}} _ {\ mathrm {ads}} + {\ mathrm {H}} ^ {+} + {\ mathrm {e}} ^ {\ hbox {-}} $$ (7) $$ \ mathrm {Ru} + {\ mathrm {H}} _ 2 \ mathrm {O} \ to \ mathrm {Ru} \ hbox {-} {\ mathrm { OH}} _ {\ mathrm {ads}} + {\ mathrm {H}} ^ {+} + {\ mathrm {e}} ^ {\ hbox {-}} $$ (8) $$ \ mathrm {Pt } \ hbox {-} {\ mathrm {CO} \ mathrm {H}} _ {\ mathrm {anuncios}} + \ mathrm {Ru} \ hbox {-} {\ mathrm {OH}} _ {\ mathrm {anuncios }} \ to \ mathrm {Pt} + \ mathrm {Ru} + {\ mathrm {CO}} _ {2+} 2 {\ mathrm {H}} ^ {+} + 2 {\ mathrm {e}} ^ {\ hbox {-}} $$ (9) $$ \ mathrm {Pt} \ hbox {-} {\ mathrm {CO}} _ {\ mathrm {ads}} + \ mathrm {Ru} \ hbox {-} {\ mathrm {OH}} _ {\ mathrm {ads}} \ to \ mathrm {Pt} + \ mathrm {Ru} + {\ mathrm {CO}} _ 2 + {\ mathrm {H}} ^ {+} + { \ mathrm {e}} ^ {\ hbox {-}} $$ (10)En referencia a este mecanismo bifuncional, el metanol se disocia y adsorbe inicialmente en Pt, y luego se descompone en CO ads y / o especies similares al formilo -CHO ads por reacción de deshidrogenación (7). Al mismo tiempo, el agua se disocia en anuncios OH y adsorbido en sitios Ru (8). Luego, las especies se adsorben en los sitios de Pt y Ru y se combinan para formar CO 2 molécula (9) y (10). La reacción entre los anuncios de Pt – CO y Ru– OHads conduce a CO 2 evolución, generando sitios Pt y Ru renovados (reacción 10). Considerando que, otro trabajo realizado por Ewelina Urbanczyk et al. [48] llevó a cabo la reacción de oxidación de metanol para el catalizador de PtNi en medio alcalino (KOH 1,0 M). Teóricamente, la reacción de oxidación del metanol en medio alcalino es:
$$ {\ mathrm {CH}} _ 3 \ mathrm {OH} +6 \ mathrm {OH} \ to {\ mathrm {CO}} _ 2 + 5 {\ mathrm {H}} _ 2 \ mathrm {O} +6 { \ mathrm {e}} ^ {\ hbox {-}} $$La reacción se inicia en el electrodo de Pt del DMFC a dióxido de carbono. Durante este proceso, se pueden formar moléculas intermedias (CO) que pueden causar envenenamiento y desactivación del lado activo del Pt. Esta molécula de CO es producto de la oxidación incompleta del metanol. La oxidación incompleta del metanol forma el CO como producto intermedio (Ec. 11). La superficie del electrocatalizador también puede adsorber grupos hidroxilo (Ec. 6). Finalmente, debido a la desorción del producto principal, se forma dióxido de carbono (13). El segundo veneno que puede producirse durante la oxidación del metanol es el metano. En este caso, puede ocurrir la siguiente reacción (8). La oxidación total de la forma intermedia de carbono a dióxido de carbono en la reacción electroquímica es la siguiente:
$$ 3 \ mathrm {Pt} + {\ mathrm {CH}} _ 3 \ mathrm {OH} \ to \ mathrm {Pt} - \ mathrm {COads} +4 {H} ^ {+} + 2 \ mathrm {Pt } + 4e - + {H} ^ 2O $$ (11) $$ \ mathrm {Ni} + {H} _2O \ to \ mathrm {Ni} - \ mathrm {OHads} + {H} ^ {+} + e - $$ (12) $$ \ mathrm {Pt} - {\ mathrm {CO}} _ {\ mathrm {anuncios}} + \ mathrm {Ni} - \ mathrm {OHads} \ to {\ mathrm {CO}} _2 + {H} ^ {+} + \ mathrm {Pt} + \ mathrm {Ni} + e- $$ (13) $$ \ mathrm {Pt} - {\ mathrm {CH}} _ 3+ \ mathrm {Pt} - H \ to 2 \ \ mathrm {Pt} + {\ mathrm {CH}} _ 4 $$ (14) $$ \ mathrm {Pt} - {\ mathrm {CH}} _ 3+ \ mathrm {Ni} {\ left (\ mathrm {OH} \ right)} _ 2 \ to \ mathrm {Pt} + {\ mathrm {CO}} _ 2+ \ mathrm {Ni} +5 {H} ^ {+} + 5e- $$ (15)Actualmente, los investigadores todavía están estudiando técnicas de aleación para mejorar la actividad catalítica de los electrocatalizadores basados en Pt mediante la fabricación de aleaciones ternarias y cuaternarias de Pt, como PtRuSn [91, 92], PtRuNi [93,94,95], PtRuMo [70, 96 , 97], PtRuOsIr cuaternario [79, 80] y PtRuIrSn [97, 98], debido a su excelente comportamiento en MOR y eliminación de las especies intermedias (CO) que se forman en el sitio de superficie de Pt. Pero aún se desconoce la adición del tercer y cuarto metales en estos catalizadores ternarios y cuaternarios. Además, existen algunas limitaciones y desafíos en la producción de aleaciones ternarias y cuaternarias. La optimización de la morfología del catalizador y las composiciones del catalizador se vuelve difícil de obtener debido a las muchas combinaciones posibles de metales y composiciones. Sin embargo, muchos estudios demostraron que la adición de un tercer y cuarto metal mejoró notablemente la actividad catalítica, aumentó la estabilidad del catalizador y una buena tolerancia al CO hacia la electrooxidación de metanol y las aplicaciones de DMFC.
Tsiouvaras y col. [99] realizó la medición electroquímica de catalizadores PtRuMo / C y encontró que aunque todos los catalizadores ternarios eran más activos hacia la oxidación de CO y metanol que el catalizador binario, el catalizador tratado con H 2 mostró un rendimiento mejorado en aproximadamente un 15% con respecto a los catalizadores ternarios tratados en He o sin tratamiento. En 2012, Hu et al. [100] sintetizó con éxito un electrocatalizador bimetálico superior (PtNi), a saber, nanoesferas de PtNi mesoporosas huecas (HMPNN). El catalizador exhibió un desempeño catalítico sobresaliente en el MOR con una eficiencia de utilización de Pt significativamente mejorada debido a la estructura única de los HMPNN y su gran área de superficie electroquímica. Mientras que alrededor de 2016, el trabajo realizado por Yang et al. [101] también investigó la reactividad de los electrocatalizadores de PtFe bimetálicos sintetizados en los que observaron que la fuerte interacción entre el Pt y los metales de hierro (Fe) puede disminuir las energías de adsorción de los NP bimetálicos. También encontraron que las nanopartículas bimetálicas de PtFe prefieren ser adsorbidas en el grafeno de vacante única a través de los átomos de Fe cuando los átomos de Pt y Fe están en las superficies, porque las interacciones entre los átomos de Fe y el grafeno de vacante única son más fuertes que las que existen entre los átomos de Pt y grafeno de vacante única. La Figura 3 ilustra la posición de las partículas de Pt y Fe dispersas sobre el soporte de grafeno como sugirieron Yang et al. [101].
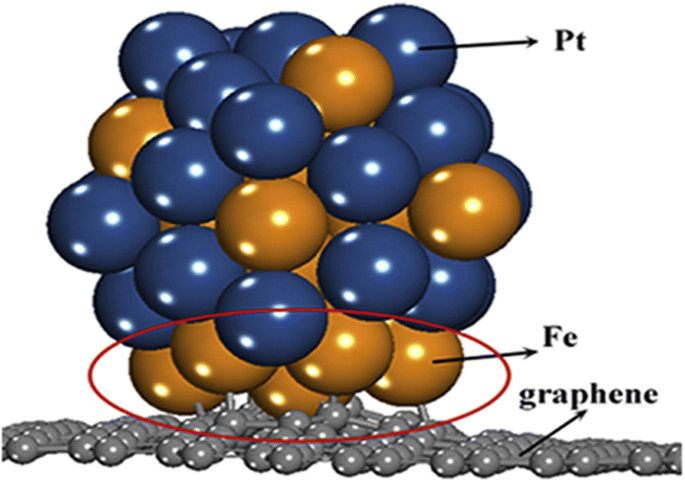
La posición del catalizador de PtFe en el soporte de grafeno ilustrada por Yang et al. [101]
Rendimiento del catalizador basado en Pt y carburo de metal de transición
El carburo de metal de transición (TMC), con alta estabilidad mecánica y química contra la corrosión, buena resistencia a ambientes ácidos, estabilidad a largo plazo y alta tolerancia al CO, puede actuar como catalizador de ánodo [88,89,90, 102,103,104]. Además, los TMC también brindan muchas ventajas en comparación con sus metales originales con respecto a la actividad, selectividad y resistencia al veneno, por ejemplo, el carburo de tungsteno (WC) muestra propiedades especiales, como buena conductividad eléctrica, resistencia a ambientes ácidos, bajo costo. y tolerancia al envenenamiento por CO en el proceso de electrooxidación del metanol [88, 105, 106].
Wang y col. [103] informó la síntesis de una gran superficie (256 m 2 g −1 ) microesferas de carburo de tungsteno mediante un método hidrotermal simple. W 2 C se encontró como la fase principal en la muestra sintetizada. Actualmente, los investigadores están explorando el potencial de Pt soportado en WC como un catalizador ideal para DMFC [38, 88, 107, 108]. Christian y col. [106] concluyó que, en relación con sus elementos de metales de transición, las TMC se comportan como metales nobles como Pt, Pd, Rh y Ru para ciertas reacciones químicas y electroquímicas, incluida la reacción de oxidación de hidrógeno, monóxido de carbono y alcohol y reducción de oxígeno. [109, 110]. En otro estudio, Liu et al. [107] presentó que los carburos de molibdeno (Mo-Carburos) podrían actuar como promotores del carburo de tungsteno y aumentar la actividad electrocatalítica en el DMFC. Sin embargo, sin la incorporación de metal Pt, la actividad electrocatalítica del WC puro hacia el MOR para un sistema DMFC sigue siendo baja. Por lo tanto, una pequeña cantidad de platino metálico agregada al componente WC es muy conveniente para obtener la ventaja del efecto sinérgico entre Pt y WC [91, 111, 112]. Mientras tanto, Hassan et al. [109] reveló que la impureza común (especies de CO) que se forma durante la oxidación del metanol tiene una fuerte energía de unión en la superficie de Pt; por lo tanto, debe oxidarse para que pueda eliminarse de los sitios activos de Pt. La adición del componente WC en el electrocatalizador Pt / WC ha mostrado una alta tolerancia al CO para MOR, lo que indica la existencia de efectos sinérgicos entre el metal Pt y el WC como componente de soporte. El estudio también fue realizado por otro investigador utilizando menos metal Pt para reducir el costo del Pt, mientras se mantiene un buen rendimiento electrocatalítico.
Aparte de eso, el componente WC es más activo para la formación de un grupo metoxi (CH 3 O-) que el Pt puro [113, 114]. El rendimiento CV de (Pt:Ru) 4-WC / RGO muestra un rendimiento catalítico excepcional con una densidad de corriente de 330,11 mA mg −1 Pt en comparación con los otros cinco catalizadores, lo que indica que el electrocatalizador sintetizado tiene una excelente actividad catalítica hacia el MOR. Además, la combinación de Ru y WC en el catalizador de Pt aumentó la cantidad de superficie de OH y permitió que el CO adsorbido en la superficie de Pt se oxidara a potenciales más bajos [39].
Rendimiento del catalizador basado en Pt y el nitruro de metal de transición
El nitruro de metal de transición (TMN) es un candidato ideal como soporte de catalizador de Pt debido a su buena conductividad eléctrica (metálica), dureza, alta estabilidad electroquímica y resistencia a la corrosión en las condiciones de funcionamiento de la pila de combustible [115,116,117,118]. Se han informado nitruros de metales de transición, como CrsN, TiN y VN, como catalizador de Pt soportado y mostraron un alto rendimiento catalítico y una mejor estabilidad en comparación con los soportes de carbono tradicionales [112]. Todos los metales de transición pueden formar nitruro, excepto la segunda y tercera fila de los metales de los grupos 8, 9 y 10 (Ru, Os, Rh, Ir, Pd y Pt). El comportamiento y las propiedades estructurales de los nitruros de metales de transición se pueden encontrar en la bibliografía [92,93,94]. Xiao y col. [112] preparó un electrocatalizador de Pt soportado con nitruro de cobalto de titanio que mostró un excelente rendimiento y estabilidad frente a la reacción de reducción de oxígeno (ORR). El Ti 0.9 Co 0.1 El catalizador de Pt soportado en N presentaba un tamaño de partícula pequeño y una buena dispersión del metal. Este electrocatalizador preparado también mantuvo el área de superficie electroquímica (ECSA) de Pt y mejoró en gran medida la conservación de ECSA, con solo una disminución del 35% en la caída de ECSA temprana después de 10,000 ciclos de ADT. El dopaje con cobalto mejoró significativamente la actividad y la durabilidad de la ORR. Mientras tanto, se puede obtener un electrocatalizador duradero y de alto rendimiento en el sistema DMFC utilizando un electrocatalizador de Pt (Ru) / TiN de gran superficie, que también demostró una alta actividad electroquímica hacia MOR con una mejora de ∼ 52% en la actividad catalítica y una buena estabilidad / durabilidad en comparación al comercial JM-Pt (Ru). Mientras tanto, el rendimiento de celda única del DMFC logró una mejor densidad de potencia máxima en un 56% y demostró una estabilidad electroquímica excepcional para el electrocatalizador CSG-Pt (Ru) / TiN [115].
Li et al. [116]. Mostró un aumento significativo en la actividad electrocatalítica hacia el MOR en condiciones ácidas y tuvo una mejor durabilidad. Las razones de estas propiedades se debieron a su trabajo de datos experimentales que verificaron que la adición de Fe puede sintonizar la estructura electrónica de los átomos de Pt, lo que contribuye a la actividad reforzada y la estabilidad del catalizador de Pt para el MOR. Mientras tanto, en trabajos anteriores realizados por Xiao et al. [117], Pt / Ti 0.8 Mo 0.2 El catalizador N presentaba una estructura porosa y nanopartículas de Pt de gran superficie, de pequeño tamaño y bien dispersas. Este sistema de catalizador conservó la estabilidad electroquímica intrínseca de la nanoestructura de TiN y mejoró notablemente la actividad y durabilidad de MOR. Sin embargo, la información disponible actualmente sobre la estabilidad electroquímica del nitruro de tungsteno (WN) todavía es insuficiente [109].
Mientras tanto, MoxN ( x =1 o 2) sobre sustrato de Ti mostró estabilidad electroquímica en un electrolito ácido de 4,4 M H 2 SO 4 hasta un potencial anódico de + 0,67 V (frente a SHE) durante 50 ciclos repetidos [110]. Sin embargo, este electrocatalizador mostró daño en la superficie, como agrietamiento y desmoronamiento, en las regiones de potencial alto catódico (por debajo de - 0,1 V frente a SHE) y anódico (por encima de + 0,67 V frente a SHE) debido a la corrosión catódica y anódica, respectivamente. En la región de alto potencial anódico por encima de + 0,67 V (frente a SHE), la composición del oxígeno aumentó debido a la formación de óxido MoOx, que podría provocar la desactivación. Estos resultados indican que MoxN reacciona con las especies de oxígeno presentes en el electrolito acuoso y es inestable por encima de + 0,67 V (frente a SHE). Mustafha y col. [111] encontró que el Pt cargado en TiN como soporte mostraba electroactividad para la oxidación del metanol, con una alta relación If / Ib que representa una alta resistencia al CO en el voltamograma realizado a una velocidad de barrido de 20 mV / s en 0,5 M CH 3 OH + 0,5 M H 2 SO 4 como el electrolito. El efecto bifuncional entre Pt y TiN se citó como la causa de la resistencia al CO de Pt / TiN. Además, Ottakam Thotiyl et al. [91] logró buenos resultados para un catalizador de TiN cargado con Pt, mostrando muy buena tolerancia al CO para la oxidación electroquímica del metanol. Concluyeron que las características especiales del TiN que lo hicieron adecuado como soporte de Pt para el MOR en un medio alcalino son que muestra una estabilidad excepcional, una resistencia extrema a la corrosión, una buena conductividad electrónica y un fuerte comportamiento de adhesión. Los catalizadores con soporte de TiN son beneficiosos en términos de estabilidad a largo plazo, densidad de corriente de intercambio y corrientes estables a bajo sobrepotencial. En los experimentos se utilizaron cargas de platino del 40% en peso sobre TiN.
En los últimos años, Liu et al. [118] successfully synthesized platinum on titanium nickel nitride decorated 3D carbon nanotubes which reduced graphene oxide (TiNiN/CNT-rGO) support by solvothermal process followed by nitriding process. Pt with small particle size is well-dispersed on TiNiN/CNT-rGO support. The 3D shape of CNT-rGO support gives a fast route for charge transfer and mass transfer as well as TiNiN NPs with good synergistic effect and the strong electronic coupling between different domains in TiNiN/CNT-rGO support. Thus, it greatly improved the catalytic activity of this catalyst. In another research, the non-carbon TiN nanotubes-supported Pt catalyst done by Xiao et al. [119] also displayed enhanced catalytic activity and durability toward MOR compared with the commercial Pt/C (E-TEK) catalyst.
Performance of Pt-Based Catalysts with Transition Metal Oxide
Pan et al. [92] reported the synthesis of platinum–antimony-doped tin oxide nanoparticles supported on carbon black (CB) as anode catalysts in DMFC, which exhibited better improvement in catalytic activity toward MOR compared to Pt-SnO2 /C or commercial Pt/C electrocatalyst. The enhancement in activity was attributed to the high electrical conductivity of Sb-doped SnO2 , which induced electronic effects with the Pt catalysts. Another work done by Abida et al. [93]described the preparation of Pt/TiO2 nanotube catalysts for methanol electrooxidation. El TiO 2 nanotubes-supported Pt catalyst (Pt/TiO2 nanotubes) exhibited excellent catalytic activity toward MOR and had good CO tolerance. They also reported that the use of hydrogenotitanate nanotubes as a substrate for the Pt catalyst considerably improved the COads oxidation on Pt, but the MOR still occurred at high potential. Then, several years later, Wu et al. [94] synthesized Pt-C/TiO2 with MOR activity 1.6 higher than commercial Pt-C and the stability of Pt-C/TiO2 was also enhanced by 6.7 times compared to Pt-C. The excellent performance of this catalyst was a contribution of mesopores and partially coated carbon support. Zhou y col. [95] prepared hollow mesoporous tungsten trioxide microspheres (HMTTS) using the spray-drying method to yield Pt/HMTTS. The electrocatalyst exhibited excellent electrocatalytic activity and high stability toward MOR than Pt/C and Pt/WO3 electrocatalysts, which may be attributed to the well-ordered Pt particles (with an average size of 5 nm) on the HMTTS surface. Wu y col. [120] used polystyrene spheres as templates to obtain pore-arrayed WO3 (p-WO3 ). The Pt nanoparticles with an approximate size of 3.3 nm dispersed on pore-arrayed WO3 (Pt/p-WO3 ) exhibited high catalytic activity toward MOR.
Li y col. [121] used Sn-doped TiO2 -modified carbon-supported Pt (Pt/Ti0.9 Sn0.1 O 2 –C) as an electrocatalyst for a DMFC system. The synthesized Pt/Ti0.9 Sn0.1 O 2 –C electrocatalyst revealed high catalytic activity and CO tolerance toward MOR. The enhanced catalyst activity was due to the high content of OH groups on the Ti0.9 Sn0.1 O 2 electrocatalyst sample and the strengthened metals and support interactions. In addition, Lv et al. [122] also reported in their work that the addition of TiO2 could not only facilitate CO removal and hinder CO formation on Pt surface during methanol oxidation, but it can also prevent the agglomeration and corrosion of Pt, which can be concluded from strong metal-supports interaction between TiO2 –C and Pt. Huang et al. [123] revealed that a TiO2 -coated carbon nanotube support for Pt electrocatalysts could be prepared via a one-step synthesis. Hao et al. [124] developed a new catalyst composed of Pt nanoparticles deposited on graphene with MoO3 . These catalysts exhibited high catalytic activity toward MOR and high resistance to CO species. However, the size of MoO3 must be tuned by controlling the metal oxide loading.
The selection of metal oxide such as MnO, RuO, CeO, SnO2 , MgO, and V2 O 5 as additional component in electrocatalyst of Pt because of their low cost, good electrochemical properties, and have proton-electron intercalation properties [125]. From the catalytic activity aspect, it can be summarized that the addition of these metal oxides can enhance the electrocatalytic activity of DMFC and other fuel cells. The incorporation of these conducting metal oxides together with Pt catalyst could also facilitate the oxidation process of CO intermediate molecules. Hence, these types of metal have high potential to be used together with platinum as anode electrode.
Carbon support
To improve the utilization of the Pt catalysts, the carbon support is also another useful approach to be used together with Pt. Carbon materials are largely used as catalyst support because of its special properties such as relatively stable in both acid and basic electrolyte, good conductivity, and provide high surface area for dispersion of metal catalyst. It is believed that carbon materials have a strong effect that can influence the electrocatalysts properties such as metal particle size, morphology, metal dispersion, alloyed degree, and stability. Carbon supports can also affect the performance of supported catalysts in fuel cells, such as mass transport and catalyst layer electronic conductivity, electrochemical active area, and metal nanoparticle stability during the operation.
Currently, a great concern of the development in the nanotechnology field, especially carbon nanomaterials synthesis, is to create more stable and active supported catalysts. Support materials of nanoparticles are believed to be the most promising materials for catalytic activity in fuel cells, including the DMFC system. Pt has been traditionally used as nobel catalysts for many fuel cells application [126,127,128]. However, the high cost and low reserve are hindering commercialization of fuel cells and driving researchers to make the utmost of the catalyst. According to this problem, the major effort has been done toward nanoscaling of the catalyst nanoparticles to form more active sites per mass unit. The morphology, structure, and activity of the catalyst, and correspondingly the whole lifetime of a cell, thus strongly depend on the catalyst support [129]. Table 2 shows the preparation, physical properties, performance, and activity of Pt-based supported carbon done by groups of researchers. The details of Pt-based supported carbon will be performed in the following sections:“Graphene Support” to “Carbon Nanocoils”.
Graphene support
Graphene has many extraordinary properties; it exists as a two-dimensional carbon (2-D) form, which is called a crystalline allotrope, one-atom-thick planar flat sheet of sp2 tightly bonded carbon atoms with a thickness of 0.34 nm. Its carbon atoms are packed in a regular atomic-scale chicken wire (hexagonal) pattern [92, 119]. The theoretical specific surface area of graphene is 2630 m 2 g −1 , which is much larger than that of carbon black (typically less than 900 m 2 g −1 ) and carbon nanotubes (100 to 1000 m 2 g −1 ) and similar to that of activated carbon [130]. Graphene has high potential as a metal support [131, 132, 133] [33] due to its high surface area [134] for better catalyst/metal dispersion [135], high electrical conductivity [136], and good thermal properties [137, 138]. Moreover, the functionality of graphene support can be modified by changing it surface structure, and hence contribute to its potential applications, such as in fuel cells, energy storage, electrochemistry, supercapacitors, and batteries [138,139,140,141,142]. Figure 4 illustrates the preparation steps to obtain the graphene nanosheets (GNS), while Fig. 5 shows their TEM images [143]. It can be clearly observed that the thickness of the GO with many typical wrinkles obviously decreases compared to graphite. This can be explained by the presence of the rich oxygen-containing functional groups over the surface of GO [132]. Besides, both resulting GN-900 and GN-900-C contained of a large size of nanosheets structure, but the GN-900-C comprised more transparent than the GN-900.
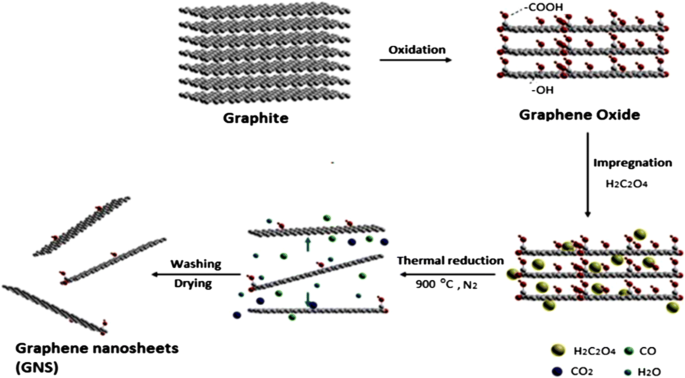
illustration of the preparation of graphite oxide to graphene nanosheets (GNS) by using oxalic acid [143]
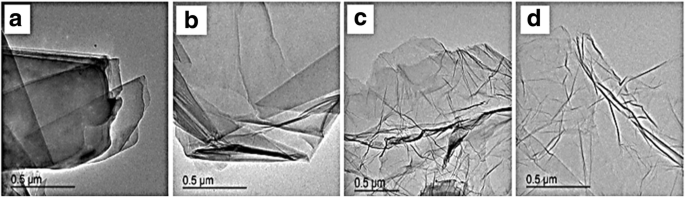
TEM images of graphite (a ), GO (b ), GN-900 (c ), and GN-900-C [143]
The discovery of graphene sheets began around 2000 by mechanical extracting process from 3D graphite source [133]. Graphene can be obtained by several synthesis methods such as hydrothermal [144], chemical reduction [143], chemical vapor deposition, and electrochemical. Ma et al. [145] enhanced the electrocatalytic activity of Pt nanoparticles by supporting the Pt nanoparticles on functionalized graphene for DMFC. Functionalized graphene was prepared by methyl viologen (MV) and Pt/MV–rGO electrocatalyst was synthesized by a facile wet chemical method. They also reported that the higher catalytic activity of Pt/MV–RGO was attributed to the synergetic effect between MV and rGO.
Meanwhile, Zhang et al. [146] modified the graphene support with graphene nanosheets through Hummer’s method, followed by polymerization of aniline (as nitrogen source). The TEM images for Pt/NCL-RGO and Pt/RGO electrocatalysts show that the aggregation between separated graphene sheets was decreased by nitrogen-doped carbon layer (NCL), leading to a better dispersion of the Pt catalyst on the graphene nanosheets support and better electroactivity and stability toward methanol electrooxidation (MOR). Presence of NCL successfully prevented the aggregation of graphene nanosheets as the Pt nanoparticles supporting material.
In 2011, Qiu et al. [135] successfully synthesized nanometer-sized Pt catalyst via sodium borohydride reduction method with an average particle size of only 4.6 nm. These Pt nanoparticles showed an even dispersion of Pt catalyst on graphene oxide support and very high electrocatalytic activity toward MOR by controlling the percent deposition of Pt loaded on the graphene. In another study conducted by Ojani et al. [147], for synthesized Pt-Co/graphene electrocatalyst, it was shown that graphene nanosheets improved the electrocatalytic behavior and long-term stability of the electrode. In addition, the Pt-Co/G/GC electrocatalyst showed great stability toward MOR. The catalytic performance toward MOR can also be improved by using cobalt core–platinum shell nanoparticles supported on surface functionalized graphene [148]. This enhanced catalytic activity could be attributed to the poly (diallyldimethylammonium chloride) (PDDA) that plays a crucial role for dispersion and stabilization of Co@Pt catalyst on graphene support. PDDA-functionalized graphene provided the higher electrochemical active surface area [149, 150]. Huang et al. [138] also studied a PtCo-graphene electrocatalyst with outstanding catalytic performance and high CO tolerance toward the MOR, which far outperformed Pt-graphene and PtCo-MWCNT electrocatalysts with the same ratio of Pt and carbon content. Figure 4 shows the formation of a graphene-PtCo catalyst prepared from a graphite source. Sharma et al. [57] synthesized Pt/reduced graphene oxide (Pt/RGO) electrocatalyst using a microwave-assisted polyol process, which sped up the reduction of GO and formation of Pt nanocrystals. They compared Pt/RGO to a commercial carbon support (Pt/C), which exhibited high CO tolerance, high electrochemically active surface area, and high electrocatalytic activity for the MOR. In a previous study, Zhao et al. [139] reported that the unique 3D-structured Pt/C/graphene aerogel (Pt/C/GA) exhibited greater stability toward MOR with no decrease in electrocatalytic activity. Moreover, the Pt/C/graphene aerogel also exhibited significantly higher stability to scavenge crossover methanol at high potential in an acidic solution compared with the commercial Pt/C electrocatalyst. At the initial catalytic stage, the Pt/C electrocatalyst lost approximately 40% after 1000 CV cycles. In contrast, the Pt/C/graphene aerogel only lost 16% of the initial catalytic activity. After 200 cycles of CV, the current density of Pt/C/graphene aerogel was much higher with a remarkably higher stability than that of Pt/C electrocatalyst. Meanwhile, Yan et al. [151] demonstrated highly active mesoporous graphene-like nanobowls supported Pt catalyst with high surface area of 1091 m 2 g −1 , high pore volume of 2.7 cm 3 g −1 , and average pore diameter of 9.8 nm obtained by applying a template synthesis method. In addition, the Pt/graphene bowls also achieved high performance toward MOR with a current density value of 2075 mA mgPt −1 , which was 2.87 times higher than that of commercial Pt/C (723 mA mgPt −1 ). The onset potential for the Pt/graphene bowls toward methanol electrooxidation was negatively shifted by approximately 160 mV compared with that to the latter and showed CO resistance. Figure 6 shows the proposed schematic for the formation of PtCo catalyst on reduced-GO (rGO) support [51]. It is described that the formation of graphene oxide nanosheets from oxidation of graphite powder leads to the increase in interlayer “d” spacing of stacked graphitic sheets from 0.34 to 0.78 nm due to the presence of various oxygen-containing functional groups. The oxygen-containing functional groups act as anchor sites for the well-dispersed Pt and PtCo nanoparticles on rGO sheets, and used for efficient electrooxidation of methanol.
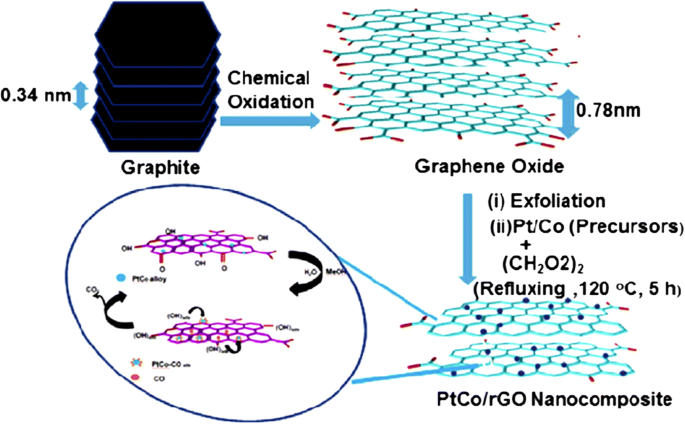
Illustrates the schematic formation of graphene supported Pt-Co catalyst [51]
We can conclude that the reduce graphene oxide (rGO), graphene, modified graphene as supporting material exhibited high electrocatalytic activity toward methanol electrooxidation process. A lot of studies have been reported related to the particle size distribution and size, morphologies, and catalytic activities of Pt and Pt alloys using graphene as supporting material, which showed great improvement in fuel cell performance as mentioned and discussed above. Thus, graphene support can be further studied for better fuel cell performance.
Multiwall Carbon Nanotube and Single-Wall Carbon Nanotube Support
Several years ago, Jha et al. [140] prepared multiwall carbon nanotube (MWCNTs) via chemical vapor deposition using an AB3 alloy hydride catalyst. Platinum-supported MWCNT (Pt/MWCNT) and platinum-ruthenium-supported MWCNT (Pt-Ru/MWCNT) electrocatalysts were prepared by chemical reduction. The performance of these electrodes was studied at different temperatures, and the results demonstrated a very high power density of 39.3 mW cm −2 at a current density of 130 mA cm −2 , which could be attributed to the dispersion and accessibility of the MWCNT support and Pt-Ru in the electrocatalyst mixture for the methanol oxidation reaction. This was also done by other researchers that using different catalyst supported MWCNT for DMFC system [152,153,154,155]. Meanwhile, Wu and Xu [156] compared MWCNT-supported Pt and single-wall carbon nanotube (SWCNT)-supported Pt. Figure 7 shows that the TEM images of Pt catalyst was deposited on MWNT and SWNT electrodes through the electrodeposition technique. The Pt particles in Pt-SWNT (Fig. 7b) looked closer contact with the network of entangled and branched bundles of SWNT support, and the shape is closer to highly exposed sphere. The benefits of the SWCNT support are due to its greater electrochemical surface-active area and easier charge transfer at the electrode/electrolyte interface because of the graphitic crystallinity structure, rich amount of oxygen-containing surface functional groups, and highly mesoporous and unique 3D-structure of SWNT. The electrodeposition technique carried out by them contributed to higher utilization and more uniform dispersion of Pt particles on its support.
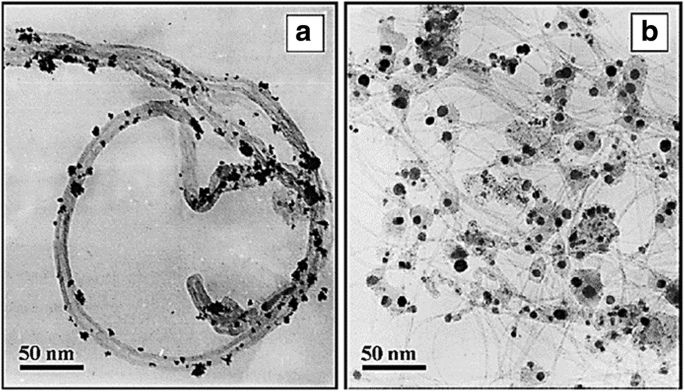
TEM images for the Pt on MWCNT(a ) and SWCNT (b ) [156]
Then, Wang et al. [157] reported the high performance of modified PtAu/MWCNT@TiO2 electrocatalyst prepared via deposition-UV-photoreduction for DMFC, which also exhibited high CO tolerance. Zhao y col. [126] studied 3D flower-like platinum-ruthenium (PtRu) and platinum-ruthenium-nickel (PtRuNi) alloy nanoparticle clusters on MWCNTs prepared via a three-step process, and the best ratios obtained from their experiments for the PtRu and PtRuNi alloys were 8:2 and 8:1:1, respectively. Another group, i.e., Zhao et al. [158], found a higher current density toward MOR and better activity for MWCNT-supported PtWC compared with Pt/C electrocatalyst. These results were attributed to the factors of the synergistic effect between the Pt catalyst and the WC component, high CO tolerance from the bifunctional effect of the Pt catalyst and the WC component, and strong interaction between metals and WC in the electrocatalyst composite.
As a summary, both of MWCNT and SWNT support in terms of structural, surface, and electrochemical properties have their own characteristics as supporting material that remarkably enhanced their performance in catalysis of methanol oxidation process. However, as a comparison, SWCNT possess a high degree of graphitization, highly mesoporous 3D structure, and contain more oxygen-containing functional groups at its surface sites. In relation with these properties, the SWCNT exhibits a higher electrochemically accessible surface area and faster charge transfer rate at the electrode/electrolyte interface.
Carbon Nanotube Support
Wen y col. [144] proposed that carbon nanotubes (CNTs) support could improve fuel cell performance; for example, Pt can be fixed to the inner wall and the outer wall of CNTs and may cause improvement in the electrocatalytic properties of platinum-CNTs. Yoshitake et al. [159] proposed that fuel cells using CNTs as the catalyst support produced larger current densities. The addition of binary or other components to the electrocatalysts for methanol electrooxidation overcomes the problems related to catalyst poisoning caused by CO during the reaction. Therefore, new electrocatalyst carbon supports, such as carbon nanotubes [160, 161], are being actively developed to significantly improve fuel cell performance. Kakati et al. [128] successfully synthesis the PtRu on CNT/SnO2 for anode catalyst DMFC via hydrothermal process. It has been found that the presence of SnO2 provide a high durability property for the catalyst and the presence of SnO2 in the district of Pt could supply oxygen-containing functional groups for the removal of CO intermediate molecules from the Pt surface sites during electrooxidation of methanol. Generally, the decomposition methanol occurs at Pt surface sites; meanwhile, the decomposition of water occurs at SnO2 surface sites to form oxygen-containing species which then react with CO intermediate molecules. However, as support material, the conductivity property of SnO2 still needs to be enhanced. Kakati et al. [128] also proposed the schematic diagram of the formation of PtRu on CNT/SnO2 composite as shows in Fig. 8, and FESEM images of CNT/SnO2 composite support (a and b) and PtRu/SnO2 /CNT composite electrocatalyst (c and d) in Fig. 9.

Illustrates the schematic diagram for the formation of PtRu/SnO2/CNT composite [128]
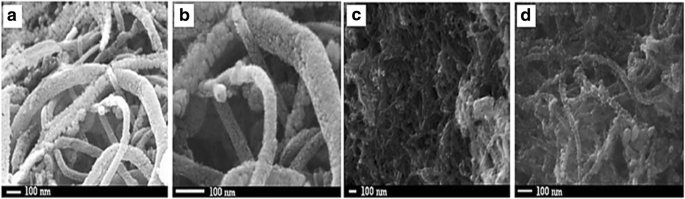
FESEM images of CNT/SnO2 composite support (a , b ) and PtRu/SnO2/CNT composite electrocatalyst (c , d )
Chien et al. [127] proposed that the high catalytic performance of Pt-Ru/CNT for MOR can be attributed to the presence of CNT as the carbon support material with several factors:(i) the as-synthesized Pt-Ru/CNT electrocatalyst owns the ideal nanosized particles and composition to increase catalytic activity, (ii) the presence of functional group on the CNT surface results in high hydrophilicity of CNT, which produces better electrochemical reaction on the electrode area, and (iii) the high electronic conductivity of the CNT support lowers the resistance in MOR. Jeng et al. [150] prepared Pt-Ru/CNT electrocatalyst via a modified polyol with a PtRu composition ratio of 1:1, exhibiting high catalytic activity toward MOR and better performance than that of commercial PtRu/C. Show et al. [162] reported that Pt catalyst with a size of less than 10 nm can be obtained by dispersing the Pt particles on a CNT surface using the in-liquid plasma method, and excellent performance was demonstrated by the electrical power achieving 108 mW cm −2 [162]. The in-liquid plasma method was also used by Matsuda et al. [163] that can applied to obtain nanometer-sized Pt catalyst on support material that remarkably enhanced the fuel cell performance.
To be concluded, high electric conductivity, large surface area, excellent chemical and electrochemical stabilities, quasi one-dimensional structure, and good morphology as the supporting materials are the key factors of carbon nanotubes (CNTs) in enhancing the DMFC performance. In addition, carbon support materials such as CNTs which contribute a large effect on metal distribution and size have also been proven to be an essential to the electrocatalysts to achieve high catalytic activity during methanol oxidation process.
Carbon Nanofiber Support
Steigerwalt et al. [164] reported the successful synthesis of PtRu alloy that was widely dispersed on a graphene carbon nanofiber (CNF) support as an electrocatalyst in DMFC. The catalytic activity was enhanced by ~ 50% relative to that recorded for an unsupported PtRu colloid anode electrocatalyst. Meanwhile, Wang et al. [152] reported that Pt/CNF nanocomposites obtained by the reduction of hexachloroplatinic acid (H2 PtCl 6 ) precursor with formic acid (HCOOH) in aqueous solution containing electrospun CNFs at room temperature showed a higher current density than other prepared Pt/CNFs and was approximately 3.5 times greater than that of the E-TEK Pt/C electrocatalyst. Another research carried out by Giorgi et al. [153] described a CNF and bimetallic PtAu electrode with a single layer and both diffusive and catalytic functions using a decreased noble metal amount (approximately five times less) with a consequent large cost reduction. In addition, the bifunctional electrocatalytic properties were also active for the MOR on the PtAu nanoparticle catalysts [154]. Calderón et al. [155] reported PtRu/CNF prepared via reduction using sodium borohydride (NaBH4 ), methanol, and formate ions. This electrocatalyst synthesized by SFM was heat-treated (denoted as SFM TT), which improved its electrocatalytic activity during MOR. Later, Maiyalagan [165] reported that the addition of silicotungstic acid acted as a stabilizer for the PtRu particles on CNT support. The PtRu-supported CNT was prepared by microwave heating of an ethylene glycol (EG) solution of STA, H2 PtCl 6 .6H 2 O (as Pt precursor), and RuCl3 .xH2 O (as Ru precursor) with CNF suspended in the solution. The Pt and Ru precursors were loaded on CNF by conventional impregnation method. The results revealed that the PtRu nanoparticles are uniformly dispersed on carbon nanofiber support, with an average particle size of 3.9 nm enhanced the catalytic activity toward methanol electrooxidation. As a conclusion, the carbon nanotubes supporting material with high electronic conductivity and high surface area gives an advantage of better dispersion for the Pt or Pt alloys deposition. The higher the surface area of supporting material can reduce the agglomeration of metal particles on it, thus can produce better catalyst morphology for better fuel cell performance.
Mesoporous Carbon Support
Mesoporous carbon (MPC) support is another ideal candidate as an electrocatalyst support material in DMFC and fuel cell. Generally, mesoporous carbons are divided into two classes based on their structures which are ordered mesoporous carbons (OMCs), with highly ordered pore structure and uniform pore size, nonordered mesoporous carbons with irregular pores. Other than that, OPC can be produced by using high quality of SBA-15 silica and sucrose as carbon source template. To prepare the high quality of SBA-15 SBA-15 sample, triblock copolymer, EO20-PO70EO20 (Pluronic P123, BASF), as the surfactant and tetraethyl orthosilicate (TEOS, 98%, Acros) as the silica source are used, as reported by literature [166,167,168]. The synthesis of MPC starts from synthesis of SBA-15, followed by calcination process.
A well-dispersed and ultralow Pt catalyst (PtFe) supported on ordered mesoporous carbon (OMC) was prepared via a simple route and showed superior catalytic activity. The PtFe alloy with a size range of 3–5 nm was homogeneously dispersed on the CMS with a very high specific surface area of more than 1000 m 2 g −1 [169]. The incorporation of Fe was discussed in the previous section (“Performance of Various Types of Pt-Based Catalysts” section and “Performance of Pt-Based Alloys” section). The high specific surface area of mesoporous carbon support can be produced by carbonization process of a resorcinol-formaldehyde polymer with a cationic polyelectrolyte as a soft template [160]. The performance of Pt/MPC also related to the synthesis/preparation method as done by Kuppan and Selvam. Kuppan and Selvam [167] synthesized four type of Pt/mesoporous carbon by using different reducing agent which are NaBH4 , EG, hydrogen, and paraformaldehyde. From there, the Pt/mesoporous carbon synthesized using paraformaldehyde as reducing agent for showed highest current density. The highest in catalytic was attributed to the use of paraformaldehyde that gives the smallest Pt particle size (4.5 nm), and the highest ECSA (84 m 2 /g) belongs to Pt/mesoporous carbon.
Wang y col. [161] synthesized a Pt@WC/OMC electrocatalyst composite, in which the composite was platinized using a pulsed microwave-assisted polyol technique. The OMC produced in this synthesis exhibited high surface area property. The Pt@WC/OMC electrocatalyst also showed high activity, desirable stability, and CO tolerance toward MOR. In another work done by Zhang et al. [170], the ordered CMS had a unique hierarchical nanostructure (with a 3-D structure) with ordered large mesopores and macropores that facilitated the dispersion of Pt nanoparticles and rapid mass transport during the reactions.
To maximize the use of Pt particles, the support materials should have uniform dispersion, high utilization efficiency, and desirable activity and stability. Moreover, the good supporting materials must be suitable for surface chemistry, high loading of Pt dispersion, and some functional roles. Additionally, based on the previous studies as discussed above, the ordered mesoporous carbons with large pore sizes are highly desirable for fast mass transfer and, thus, enhance the catalytic activity especially in the reaction involve large reactants molecules.
Carbon Black
Carbon black (CB) is one of the commercial carbon support that has been used till now. There are many types of CB such as Vulcan XC-72, Black Pearl 2000, Denka Black, Shawinigan Black, Ketjen EC-300J, etc. [171, 172]. CB is commonly used as a carbon support material for electrocatalysts because it possesses high porosity properties, which make it suitable as a potential support material for the catalyst layer in PEMFCs and DMFCs as reported in provided literatures [173,174,175,176,177,178,179,180]. The comparison of the several carbon black support was reported by Wang et al. [181] who investigated the effect on DMFC performance using several types of carbon black such as Vulcan XC-72R, Ketjen Black EC 300J, and Black Pearls 2000 carbon black as additives/support for the Pt cathode catalyst. From the experiments, the results showed that Ketjen Black EC 300J was the most useful carbon support for increasing the electrochemical surface area and DMFC performance of the cathode catalyst.
Nowadays, CB is commercial carbon support for many fuel cell systems. Generally, it is used for the comparison with new or modified catalyst [125]. The following Table 3 summarizes the commercial carbon black and its properties for fuel cell application. There are so many modifications among carbon support materials and development of new carbon support for enhance fuel cell performance; however, commercial carbon black still is used in many fuel cell applications especially for the comparison with new or modified catalyst.
Carbon Nanocoils
Celorrio et al. [182] proposed carbon nanocoils (CNCs) as a PtRu support in their experiment, indicating that the electrocatalyst performance was strongly dependent on the synthesis method. CNC-supported electrocatalysts showed better electrochemical behavior than E-TEK electrocatalysts, and better electrocatalytic behaviors toward CO and methanol oxidation were achieved using CNC as a support material [182]. Sevilla et al. obtained highly graphitic CNCs from the catalytic graphitization of carbon spherules via the hydrothermal treatment of different saccharides which are sucrose, glucose, and starch [183]. They demonstrated that the high electrocatalytic activity of the CNCs is due to the combination of good electrical conductivity of their graphitic structure and high porosity property, which allows much less diffusional resistance of reactants/products. Two years later, Sevilla et al. [184] reported highly dispersed Pt nanoparticles on graphitic CNCs with diameters in the range of 3.0–3.3 nm and a very fine particle size distribution. The electrocatalyst possessed large active Pt surface area (up to 85 m 2 g −1 Pt), high catalytic activity toward MOR (up to 201 A g −1 Pt), and high resistance against oxidation, which was noticeably greater than that of the Pt/Vulcan electrocatalyst. Celorrio et al. [185] obtained Pt/CNC electrocatalysts via the impregnation method, which showed that a combination of Pt and CNCs facilitated the CO oxidation process.
Conductive Polymer Supports
Choi et al. [186] synthesized PtRu alloy nanoparticles with two types of conducting polymers, i.e., poly(N -vinyl carbazole) and poly(9-(4-vinyl-phenyl)carbazole), as the anode electrodes. Electrochemical and DMFC tests showed that these nanocomposite electrocatalysts were beneficial in a DMFC system, but their catalytic performance was still lower than that of a carbon supported electrode. Thus, they suggested that higher electrical conductivity of the polymer and lower catalyst loss are required in nanocomposite electrodes to achieve better performance in a DMFC. Choi et al. [171] and Kim et al. [172] prepared polyaniline (PANi) as a support material for PtRu catalyst in a DMFC system. PANi is a group of conductive polymers with high electronic conductivity and a methanol oxidation current similar to that of carbon-supported PtRu catalyst. Then, Kim et al. [172] conducted catalytic tests to compare PtRu/PANi support with PtRu/carbon support, showing that the enhanced catalytic activity of PtRu/PANi was due to (i) the high electrical conductivity of the polyaniline support, (ii) the increase of electrochemical surface area of the prepared electrocatalyst, and (iii) the higher ion diffusion behavior. In another study, Amani et al. [74] synthesized PtSn supported by C-PANI as an electrocatalyst with different Pt:Sn atomic ratios using the impregnation method. The PtSn/C-PANI electrocatalyst with a ratio of 30:70 showed outstanding performance in the methanol electrooxidation, and the current density was approximately 40% higher than PtRu/C and 50% higher than Pt/C-PANi. The CO tolerance and stability were improved compared to that of PtRu/C, and the methanol crossover was reduced. Yaldagard et al. [173] studied the electrocatalytic performance of Pt/PANi/WC/C electrocatalyst for methanol electrooxidation (MOR) and oxygen electro-reduction (ORR), and it exhibited higher MOR activity, high CO resistance, and improved stability compared to Pt/C electrocatalyst in the presence of methanol.
Wu y col. [174] presented polypyrrole nanowire networks (PPNNs) as the anodic microporous layers (MPLs) of passive DMFC. In passive DMFC system, the novel MPL achieved a 28.3% increase in the power density from 33.9 to 43.5 mW cm −2 compared with the conventional layer with a similar PtRu (1:1). The high performance was due to the presence of PPNNs, which expressively improved the catalyst utilization and mass transfer of methanol on the anode. Besides, Selvaraj and Alagar [175] prepared Pt-Ru nanoparticle-decorated polypyrrole/multiwalled carbon nanotubes (Ppy/CNT) via the in situ polymerization of Ppy on CNTs containing ammonium peroxydisulphate (NH4 )S2 O8 as an oxidizing agent at the temperature range of 0–5 °C, followed by deposition of Pt particles on PPy-CNT composite films via chemical reduction to produce Pt/PPy-CNT. It was found that the PtRu particles deposited on PPy–CNT composite films exhibited higher catalytic activity and stability toward MOR compared to Pt/PPy-CNT. So far, the investigation on polymer as supporting materials is not much as carbon support materials. From aspect as supporting materials, the performance of polymer support was not good/excellent as carbon support. Further studies are needed in the future for better electrocatalytic activity and DMFC performance.
Problems and Limitations of Using Pt for DMFC Systems
There are two major challenges in the development of new DMFC catalysts:(i) performance, including the catalytic activity, reliability, and durability; and (ii) catalyst cost reduction. Two major problems arise in DMFC when using pure Pt alone as the anode catalysts:(1) slower kinetics oxidation of methanol, even on some state-of-the-art anode catalysts, and methanol crossover through the membrane, which not only lowers cathode performance but also reduces fuel efficiency. To develop successful fuel cell technology, including DMFC technology, new catalysts must be investigated to improve the performance and reduce the cost. Reduction of the catalyst cost remains a major challenge. Currently, platinum is one of the most effective electrocatalysts for DMFC due to its high catalytic activity for methanol oxidation, but because it is a precious metal, platinum usage is challenging and limited [176, 177]. Therefore, many scientists have attempted to find materials that can behave like Pt catalysts. One problem with the MOR in DMFCs is that CO is produced as an intermediate reaction product when using Pt catalyst and has strong binding energy on platinum particles, poisoning the active sites of the platinum surface area [58]. Therefore, CO must be removed by oxidizing it from the Pt surface using another material with high resistance to CO poisoning. For example, Hwu et al. proposed Pt-modified WC catalyst that has remarkable resistance to CO poisoning [178]. On the other hand, they also suggested that CO tolerance originates from the lower CO desorption temperature on pure and Pt-modified WC compared to pure Pt.
There are many solutions that can be applied to reduce the cost of Pt, overcome or minimize the formation of CO species during methanol oxidation, and increase the kinetics of methanol oxidation, such as alloying with other metals or transition metals, the incorporation of metals, metal nitrides, and metal oxides and the use of carbon supports as discussed in this paper. However, to overcome this problem, we need to understand the formation of CO on Pt sites particle, and understanding of the mechanism of the anode reaction in DMFCs. Unfortunately, it has limited amount of mechanistic insight to be studied, because this reactions involve complex mechanism path with many possible intermediate molecules and also competing reaction pathways [179]. For Pt catalytic mechanism, it has been suggested by a direct reaction path. Unfortunately, the use of Pt on other metals has limited mechanistic information available. Figure 10 represents the reaction path for methanol electrooxidation and their possible intermediates molecules formed during the process. Black arrows show direct path, while green arrows show the indirect mechanism for CO2 formation as a final product. In the direct mechanism, the reaction path does not involve a CO intermediate, and CO2 is formed directly from methanol. In contrast, indirect mechanism forming a CO intermediate molecule and subsequently it is oxidized to CO2 product. Notably, CO is the most stable molecule of all the intermediates on Pt during MOR. The stability of CO causes it to be a main reason for the extensive CO poisoning problem that is often found on Pt catalyst.
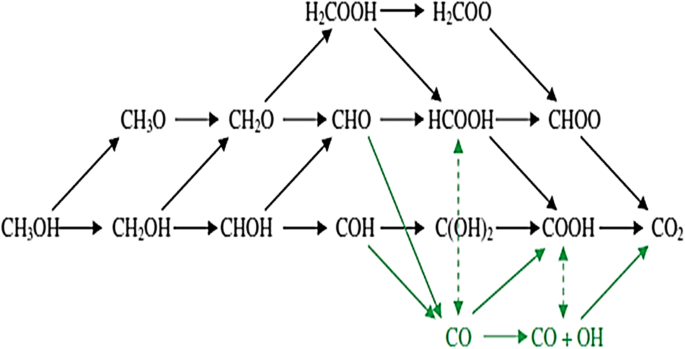
Schematic of the reaction paths and possible intermediates molecules considered in methanol electrooxidation [237]
First step in the mechanism of methanol decomposition reaction on Pt is the activation of methanol molecule. It can take place via hydrogen abstraction from either the carbon or the oxygen atoms. Further step, hydrogen abstraction creates formaldehyde (CH2 O) or hydroxymethylene (CHOH), followed by formyl (CHO) or COH. In the direct mechanism, instead of stripping off the final hydrogen from CHO or COH molecule to CO, a water molecule will release a proton/electron pair and resulting to OH species that can further bind with the carbonaceous species to form dihydroxycarbene (C(OH)2 ) or formic acid (HCOOH). This step is called hydroxyl addition process. The next step is followed by dehydrogenation to form either formate (HCOO) or carboxyl (COOH) molecule, with subsequent dehydrogenation to form CO2 as the final product of reaction. In addition, an alternative direct mechanism involve the stripping of a proton/electron pair from water and addition of the resulting hydroxyl to CH2 O, subsequently to H2 COOH, which then undergoes dehydrogenation to form HCOOH or dioxymethylene (Hs COO). The Hs COO molecule can then undergoes dehydrogenation to HCOO and finally to CO2 . Besides, in the indirect mechanism, CHO or COH species are directly dehydrogenated to CO. Water is dissociated separately on the surface to form OH, and the two surface species react together to form CO2 gas in a way similar to the water-gas-shift reaction [187]. This indirect mechanism occurs because less energy is required to form CO than CO2 . Strong adsorbed CO intermediate form on the Pt surface sites revealed a major problem at the anode site of DMFC. Formation of this intermediate species can cause deactivation Pt catalyst. Furthermore, the rate of kinetic methanol oxidation for DMFC is slower. Therefore, to increase the resistance of Pt catalyst to CO poisoning on the electrodes, Pt alloy or hybrids, such as PtRu, PtSn, PtMO, PtPb, PtFe, PtCo, PtNi, PtRuOs, PtRuMo, PtRuSn, PtRuNi, etc. (as mentioned and discussed in “Performance of various types of Pt-based catalysts” section), are usually employed as electrocatalyst materials on DMFC anodes. Addition/incorporation of these alloys to Pt can prevent the adoption of CO on Pt surface by decreasing the oxidation overpotential of the anode [84].
Conclusion and Prospects
Great progress has been made in recent years in the development and optimization of new catalysts using Pt-based catalysts and carbon and conductive polymer supports for DMFC anode catalyst. Some new carbon materials, such as nano- or mesostructured carbons, have been demonstrated as highly potential catalyst support materials, although their applications face challenges in terms of synthesis, metal loading, and electrode preparation. The combination of platinum as the best metal catalyst for DMFC and an excellent carbon support could produce breakthroughs in the investigation of a new DMFC anode catalyst in the future. Since platinum is an expensive metal, it is necessary to reduce the amount of Pt used in the electrocatalyst. Therefore, this paper presented more than 100 studies on the electrocatalytic activity and performance related to Pt-based electrocatalysts and various carbon and conductive polymer supports. The main problems related to the platinum electrocatalyst, such as carbon monoxide formation during the methanol oxidation reaction and the poor kinetics of methanol oxidation, could be overcome using additional materials and various supports, as reported in the research presented in this paper.
Many studies conducted in the recent years to reduce the loading amount of Pt catalyst and to increase the percentage utilization efficiency, and hence, enhance the electrocatalytic activity of Pt toward the oxygen reduction reaction (ORR) and methanol electrooxidation reaction (MOR), were discussed in this paper. Pt has been alloyed with many transition metals such as Fe, Co, Ni, Ir, Ru, Rh, and Pd, resulting in higher catalytic activity for the DMFC system. The incorporation of these materials also resulted in good dispersion on the carbon and polymer supports, which showed higher performance in the DMFC test compared to the use of Pt metal alone. Various carbon support sources, namely activated carbon (AC), carbon black (CB), multiwall carbon nanotubes (MWCNTs), carbon nanofibers (CNFs), carbon nanotubes (CNTs), graphene, and conductive polymer supports, have been used with Pt-based catalysts to improve their catalytic performance. Additionally, Pt-based alloy catalysts have been designed as hollow mesoporous PtNi, nanowire PtRu, and nanodendritic PtRh, which showed improved electrocatalytic activity and superior electrocatalytic performance. Meanwhile, 3-D Pt/C/graphene aerogel demonstrated enhanced stability toward methanol electrooxidation. The work performed by researchers showed that the electrocatalytic activities of nanoparticles Pt alloy catalysts depend on several factors such as the synthesis method, condition of experiments (such as temperature and pH), alloy composition/ratio, precursors, and thermal treatment. For the future study, it should be extended to the optimization of the geometry and structure of previous studies that revealed active Pt alloys can increase their electrocatalytic activity and stability and the application of support materials for fuel cell applications. For example, current research that have been done by Liu et al. 2017 [188] shows the excellent performance of platinum. From theoretical calculations, it revealed that the main effective sites on platinum single atom electrocatalysts are single-pyridinic-nitrogen-atom-anchored single-platinum-atom centers, which ascribed to the tolerant CO in MOR. They also suggested that carbon black supported used together with Pt single atom is effective in cost, efficient, and durable electrocatalyst for fuel cell application. According to the above study, herein, we can conclude that the modification on structure and morphology of precious metal such as platinum could also remarkably increase the performance of electrocatalyst, but in the same time can reduce the overall cost of fuel cell for commercialization.
To improve the morphologies of Pt and Pt alloys, carbon support material also need further study. Nanoporous metals become an interesting part of catalyst to be studied for fuel cell application. It is determined very suitable for fuel cell catalysts because they possess high surface area, three-dimensional (3D) network structures with adjustable ligament/pore sizes suitable for mass transport, and electron conduction. Around 2017, Li et al. successfully carried out modification on Pt-Pd-Au trimetallic surface as cathode for oxygen reduction reaction [189]. The surface evolution of 3-D Pt-Pd-Au trimetallic greatly enhanced the ORR activity and highly stable as ORR catalyst. The modification of PtNi alloy also done by Li et al. 2016 [190] showed ultrafine jagged platinum nanowire with highly large ECSA that exhibits enhanced mass activity of ~ 50 times higher than state-of-the-art commercial Pt/C catalyst, while Bu et al. 2016 [191] reported highly uniform PtPb/Pt core/shell nanoplate with biaxially strain extremely active, stable for anodic oxidation reactions, and great performance compared to commercial Pt/C in both methanol oxidation reaction (MOR) and ethanol oxidation reaction (EOR). Since the nanostructured platinum becomes an efficient catalyst for fuel cells as well as various industrial chemical reactions. Thus, these modifications on surface of Pt particles electrocatalysts could also to be applied in MOR for future DMFC.
On the other hand, to reduce the consumption of the Pt catalysts, the modification of the carbon support is also another useful way. This not only improves the transport capacity of protons but also reduces the usage of Nafion, which can cut the cost of the fuel cell. Moreover, with regards to the carbon support for the ORR catalysis, the hydrophobic carbon support material is required to allow water (product) to be quickly removed from the catalyst surface sites, and oxygen (reactant) to access the active sites. In contrast, the MOR catalysis requires a certain degree of hydrophilic carbon support. It can be achieved by the modification of the carbon support materials. By combination of modified carbon support materials and development of new carbon support with Pt metal catalyst, it is possible to get an ideal electrocatalysts for direct methanol fuel cell technology. Combination of Pt metal with varied carbon supports with different specific surface areas, structures, pore sizes, electronic properties, and morphologies could be great catalyst to be studied for future DMFC.
Carbon support also influence the overall performance for DMFC. Vulcan XC-72R, which is a commercial carbon support, has a large surface area, appropriate particle size, and good electrical conductivity for good support. However, in the process of depositing metal particle on these support with loading of 40% or more, the particle size of metal increased quickly, which is a disadvantage for DMFC, because a higher metal loading is used to give a better performance. In addition, multiwalled carbon nanotubes (MWCNTs) and carbon nanofibers (CNFs) with relatively smaller surface area, large diameter, and high aspect ratio could be very difficult to deposit a catalyst with high loading metal (40% and more). Therefore, modification of MWCNTs and CNFs support must be done to improve its surface area, surface functional groups, and reduce the wall thickness to achieve outstanding performance for direct methanol fuel cell even though high loading metal catalyst is consumed. As well, a great and important part to be further studied in DMFC system is about the anode and cathode catalyst preparation approaches.
Abreviaturas
- CB:
-
Carbon black
- CH3 O:
-
methoxy group
- CNC:
-
Carbon nano cage
- CNF:
-
Carbon nano fiber
- CNT:
-
Carbon nano tube
- Co:
-
Cobalt
- Co:
-
Cobalt
- CO:
-
Monoxide molecules
- CO2 :
-
Carbon dioxide
- DMFC:
-
Direct methanol fuel cell
- FC:
-
Fuel cell
- Fe:
-
Iron
- MOR:
-
Methanol oxidation reaction
- MPC:
-
Mesoporous carbon
- MWCNT:
-
Multi wall carbon nanotube
- Ni:
-
Nickel
- OMC:
-
Ordered mesoporous carbon
- ORR:
-
Oxygen reduction reaction
- PANi:
-
Polyaniline
- PEMFC:
-
Proton exchange membrane fuel cell
- Ppy:
-
Polypyrrole
- Pt:
-
Platinum
- Pt/MWCNT:
-
Platinum-supported MWCNT
- Pt-Ru/MWCNT:
-
Platinum-ruthenium-supported MWCNT
- Rh:
-
Rhodium
- Ru:
-
Ruthenium
- Sn:
-
Sternum
- SOFC:
-
Solid oxide fuel cell
- SWCNT:
-
Single-wall carbon nanotube
- TMN:
-
Transition metal nitride
Nanomateriales
- Nanopartículas de oro multifuncionales para aplicaciones terapéuticas y diagnósticas mejoradas:una revisión
- Avances y desafíos de los nanomateriales fluorescentes para síntesis y aplicaciones biomédicas
- Compuestos de grafeno y polímeros para aplicaciones de supercapacitores:una revisión
- Fabricación y caracterización de un nuevo catalizador anódico compuesto de nanofibras de carbono Tio2 para celdas de combustible de metanol directo mediante el método de electrohilado
- Rendimiento mejorado de un nuevo catalizador anódico de PdAu / VGCNF para la electrooxidación en una pila de combustible de glicerol
- Evaluación de estructuras de grafeno / WO3 y grafeno / ceO x como electrodos para aplicaciones de supercondensadores
- Soporte de catalizador anódico novedoso para pila de combustible de metanol directo:caracterizaciones y rendimiento de una sola pila
- Aplicaciones biomédicas para nanoclusters de oro:desarrollos recientes y perspectivas futuras
- Revisión:Membranas y filtros de metal poroso para la separación de aceite y agua
- Nanoclusores de oro de neuroproteína fluorescente:síntesis y aplicaciones en la detección de lectina vegetal y la obtención de imágenes celulares
- Solvay lanza cinta de fibra de carbono de alto rendimiento para aplicaciones de petróleo y gas en alta mar



